-
Posts
88,184 -
Joined
-
Last visited
-
Days Won
1,877
Content Type
Profiles
Forums
Gallery
Events
Everything posted by SwampNut
-
TBF, Zero is pretty much a caretaker for his mom, and that should be admired.
-
Not mocking, just a statement. If someone can't be trusted to use a power plug, they also can't use the hose or keep it charged. Just that simple. A battery powered emergency device is retarded. Also it turns out my neighbor bit on the Airmoto scam site and got the four pack, which you have to if you want a fair price. Two have failed he said. He confirmed they are the same unit as what I got. Ordered this, happened to be 40% off today. Good review on Adv Rider.
-
100%. Joe unfortunately the real issue is balancing. I'm sure I could change a dirt tire, of course, pretty sure I know where the tools are even. But not balance it. And that's what they are claiming is the delay. Today around 11 or noon I'll go there and just take back whatever they have in whatever state it's in, and go to another shop as needed.
-
Dude wut? Both of my cars came with a Slime brand kit with a sealer and a compressor that plugs into the 12v outlet formerly called a cigarette lighter. It fills the tire in about three minutes or so, as often as you need. It keeps working when frozen (batteries don't) and won't self destruct when super hot (batteries do). It doesn't need to be kept charged. Reminds me, I bet the jump pack in the Smart is dead, because well, fucking batteries. The Smart even has a place built in to hold the compressor and Slime bottle, since modern cars don't have spares. The Tesla just has it in the sub-trunk. Your friend is probably too stupid to connect the hose, or to keep the battery charged.
-
Thought of you as I stew over the second day after "it will be done in a couple hours tops." The front of the bike is in the air, I want to do things to it that require it being outside. (Aluminum cleaner, drain fuel.). I'm thinking maybe I can put the forks in my garden cart, but not sure I can get the cart around the bike to the front of it. Couple of neighbors to carry it? These fuckerheads gave me a bad feeling when I dropped off the wheel. The counter guy was pretty wasted at 10am, Snoop would have looked at him and said, "Too much bro." They used to be good. Also, I guess I should note, the shop is called "Screwie Lewie's" after all.
-
It arrived, it's retarded huge and going back. I know the listings had size, I guess it didn't really scale from numbers to how much space it wastes. It would be fine for a car but why would you ever have a battery powered compressor in a car? Dumb.
-
Yeah, no more fasteners from Amazon, ever, unless they are branded and sold direct from the brand. In my defense, I got these a while ago along with the batch of all the other bullshit from Amazon. These should get about 14-16nm of torque. I got to 7 when they did this. Now, on the other hand, I was able to get 14 from them with a better tool, these oddly shaped but obviously super effective Wera drivers. So this speaks to the advantage of good drivers too. BUT that doesn't let the fasteners off the hook because the other tool is the one I have used for years on bicycles, to over 14nm, without any issue ever. In fact I love how precise they are so they live on my work cart and get 10x more miles than any others. Oh, yeah, the Amazon fasteners have loose heads too. Almost like a Torx shape but not quite.
-
And in the bedroom.
-
Oh, guess I happened upon a deal. But there is a 12% coupon at least. Here they are with frosted lenses for $50: https://www.ebay.com/itm/305180680976?var=604113234641 I don't know if you read the air compressor thread where I described doing a google image search for product images. With Chinese commodities like these there are always multiple sources and they use the same pics, so easy to find a deal.
-
It is, exactly. Pretty common with Chinese imports, I've seen it with a lot of products. That's why I bought it, just not from the scammers.
-
Supposedly 6mm bolts. When they felt wrong going into the bike, I checked one on my hardened steel thread pitch gauges, and this is the result. Then holding them up to a normal bolt, you can see that over the length of the screw, the pitch is just a tiny bit off. WTF. Also it's truly Chinesium shit metal, soft as aluminum.
-
In one extreme case I got flexible exhaust tubing and had around a 1-foot "snorkel" up the side. You just need to find the actual flowing air, not the back pressure area.
-
With stinky vehicles in the past I've found that just a few inches of exhaust extension can make a huge difference. I've also used generic foam seals on some vehicles, there are a LOT of shapes out there. I can't remember what search I've done to find a page full of shapes to pick from. https://www.google.com/search?q=epdm+door+seal&client=safari&sca_esv=598813290&rls=en&sxsrf=ACQVn09pzTybRL-2S_dV0z80i68kZ7CGtw%3A1705418775252&ei=F6CmZcP6Dt7AkPIPrsOk4A4&ved=0ahUKEwiD4qqCnOKDAxVeIEQIHa4hCewQ4dUDCA8&uact=5&oq=epdm+door+seal&gs_lp=Egxnd3Mtd2l6LXNlcnAiDmVwZG0gZG9vciBzZWFsMgsQABiABBiKBRiRAjIGEAAYFhgeMggQABgWGB4YDzIIEAAYFhgeGA8yBhAAGBYYHjIGEAAYFhgeMggQABgWGB4YDzIGEAAYFhgeMggQABgWGB4YDzILEAAYgAQYigUYhgNImx1QkBBY0xtwAngBkAEAmAF_oAH-B6oBAzIuN7gBA8gBAPgBAcICChAAGEcY1gQYsAPCAg0QABiABBiKBRhDGLADwgIFEAAYgATiAwQYACBBiAYBkAYK&sclient=gws-wiz-serp
-
Depends. The closest one is four crow miles, but a lot farther in legal miles. I have to go WAY around a preserve to get there. The entrance is off 303. Next ones, either White Tank or the area around the lake, is quite a bit more down some highways with fast traffic (74, 303, Sun Valley Parkway). Around 30-40 minutes. I'm just not comfortable with doing it on low tires, and won't. Particularly when it starts to heat up. Oh, and these are tubed tires, they will heat up a lot. Anyway, logic aside, I'll never feel comfortable doing it, and this is about having fun. Yeah, near all of the sales blurbs are useless bullshit. The one I bought had some specific numbers on the time to get a sport bike tire from 0-30, and that was useful (and now I forgot the number, but not bad). One said it could air up tires fast because it had a "high performance chip." WTF All of the add-on pack options were much larger than the dedicated units. And almost as expensive. So now...starting pressures. I'm thinking 30f 33r for street and 13/13 dirt.
-
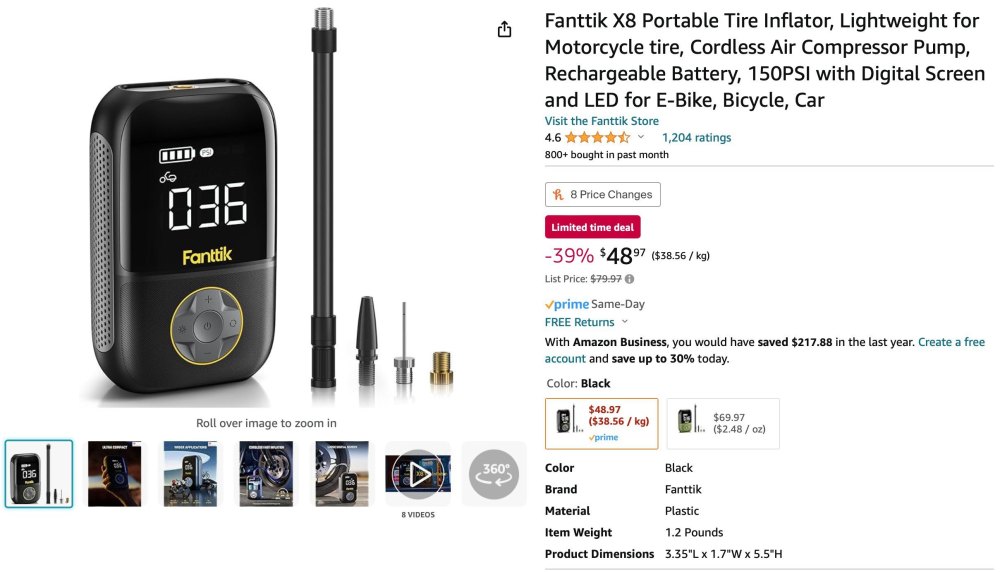
I have those on the bicycles, cost adds up fast, you have to carry a bunch for a motorcycle. The Airmoto site is a scam. Did you read the site? This is why I was asking for experiences; everything is astroturfed bullshit. Anyway, I bought the exact same unit for half the price without the name on it and a longer warranty. I put the picture from Airmoto into image search, and it showed me all of the sources of the same generic Chinese unit. Amazon had the second best price and best delivery. It's the only compressor with high reviews which Fakespot rates as an A.
-
I have a few, no thanks.
-
That's the big question. I think if I don't like it, what I've done will make it a super easy, profitable sale. And I've enjoyed wrenching on it, for sure. Things I can do in a closed heated garage. Maybe it's a project for every winter. I did resist some of the higher dollar upgrades like the real charging system, and replacing the worn items that cost a lot like the case covers. If I like it, then yeah.
-
Hah, well, there's the problem. What doesn't suck? I have a plug-in Stop & Go that is awesome. Their cordless is fucking garbage.
-
https://www.amazon.com/s?k=motorcycle+rechargeable+air+compressor&i=automotive&crid=3P020Q6OUG21U&sprefix=motorcycle+rechargeable+air+compressor%2Cautomotive%2C133&ref=nb_sb_noss I use FakeSpot to let me know about fake reviews. Except now that EVERYONE is astroturfing, even good products have fake good reviews. So Fakespot lists them as F-grade, which is accurate, but the product is still perfectly fine. But vendors are all faking reviews to win. Sigh.
-
No, there are dozens with internal 18650/27 batteries or lipo flat packs. Monstrous. The difference between making it up a hill and not, by 50% at least. I've spent a lot of time on our local terrain on MTBs and motorcycles, there's simply no way you'd want to do anything more than a fire trail at street pressure. Local wisdom has us around 10-13 for motorcycles and I run 19-21 on MTB.
-
I'll be riding the XR to the trail, so I need to air down and back up. It has no useful electrical system so I need a compressor with its own battery. Shopping them is such a pain, with so much bullshit in every listing, reviews all over the place, and so many that look identical to others for 50% more or less. Anyone using one they recommend? Or hate?